SHOW NO MERCY TO IMPETIGO
ANTIMICROBIAL DATA
XEPI®(ozenoxacin) CREAM, 1% IS BACTERICIDAL VS BACTERIOSTATIC1,2
Ozenoxacin is bactericidal at low concentration against S. aureus1,2
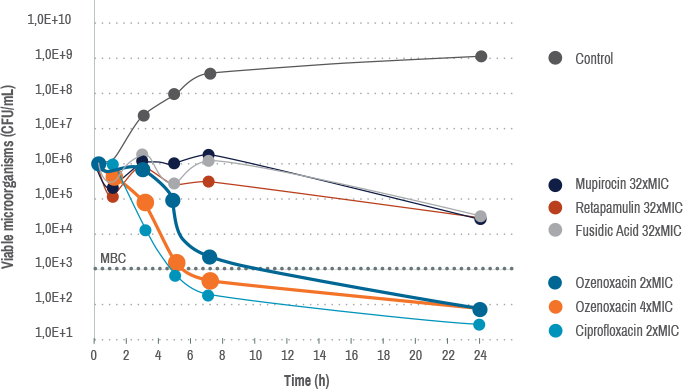
This chart shows in vitro data. In vitro data may, but does not always, correlate with clinical findings.
Potential for Microbial Overgrowth: Prolonged use of Xepi® may result in overgrowth of nonsusceptible bacteria and fungi. If such infections occur, discontinue use and institute alternative therapy.1
Bactericidal activity of ozenoxacin compared with mupirocin, retapamulin, and fusidic acid against S. aureus ATCC 6538, evaluated by kill curves.2
MBC, minimal bactericidal concentrates; MIC, minimal inhibitory concentration; CFU, colony-forming units; xMIC, number of times the MIC.
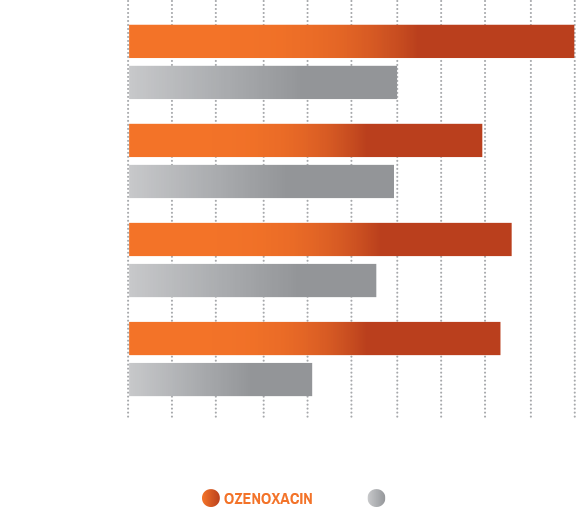
DIFFERENCES IN MICROBIOLOGICAL SUCCESS RATES BETWEEN XEPI® COMPARED TO PLACEBO ASSESSED AT 3 TO 4 DAYS IN PEDIATRIC PATIENTS STRATIFIED BY AGE3,‡
Patients were treated twice a day for 5 days
Adverse reactions (rosacea and seborrheic dermatitis) were reported in 1 adult patient treated with Xepi®.1,4
- Data for pediatric patients with impetigo (N=534) who participated in a phase 1 study (n=38) and two multicenter, randomized, placebo-controlled phase 3 clinical trials of ozenoxacin (1%) cream analyzed by age group. Although the main aim of the phase 1 study was to evaluate the systemic absorption of ozenoxacin in patients with impetigo, patients were also assessed for safety, tolerability, and clinical responsiveness3
- Microbiological success was defined as the absence of the original pathogen(s) identified in the culture of a specimen taken from the affected area at baseline with/without the presence of any new microorganisms3
- Due to the selection of pediatric patients for re-analysis and consequent decrease in statistical power relative to the entire patient population per study, comparisons by age group were analyzed descriptively3
‡The microbial success rate was 100% in the 2 to <6 months age group after ozenoxacin treatment (n=2); this was comparable to placebo (n=2)3
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION AND USAGE
Xepi® (ozenoxacin) Cream, 1% is indicated for the topical treatment of impetigo due to Staphylococcus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older.
Apply a thin layer of Xepi® topically to the affected area twice daily for 5 days. Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age.
IMPORTANT SAFETY INFORMATION
Xepi®, containing 1% ozenoxacin, is for topical use only. Not for ophthalmic, oral, intranasal or intravaginal use.
Apply a thin layer of Xepi® topically to the affected area twice daily for 5 days. Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age.
Potential for Microbial Overgrowth: Prolonged use of Xepi® may result in overgrowth of nonsusceptible bacteria and fungi. If such infections occur, discontinue use and institute alternative therapy.
Adverse reactions (rosacea and seborrheic dermatitis) were reported in 1 adult patient treated with Xepi®.
There are no available data on the use of Xepi® in pregnant women to inform a drug associated risk. No data are available regarding the presence of ozenoxacin in human milk, and the effects of ozenoxacin on the breastfed infant or on milk production.
The safety and effectiveness of Xepi® in the treatment of impetigo have been established in pediatric patients 2 months to 17 years of age. The safety profile of Xepi® in pediatric patients 2 months and older was similar to that of adults.
The safety and effectiveness of Xepi® in pediatric patients younger than 2 months of age have not been established.
Please read the US Full Prescribing Information for Xepi® available at https://www.xepicream.com/PI.
You are encouraged to report side effects of Xepi®. Please contact Biofrontera Inc. at 1-844-829-7434 or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
References: 1. Xepi® [package insert]. Woburn, MA: Biofrontera, Inc.; 2020. 2. Vila J, Hebert A, Torrelo A, et al. Ozenoxacin: a review of clinical and preclinical efficacy. Expert Rev Anti Infect Ther. 2019;17:159-68. 3. Hebert AA, Rosen T, López NA, et al. Safety and efficacy profile of ozenoxacin 1% cream in pediatric patients with impetigo. Int J Womens Dermatol. 2020;6(2):109-115. 4. Rosen T, Albareda N, Rosenberg N, et al. Efficacy and safety of ozenoxacin cream for treatment of adult and pediatric patients with impetigo: a randomized clinical trial. JAMA Dermatol. 2018;154(7):806-813.