
OVERVIEW: IMPETIGO
(IMPETIGINOUS BACTERIA)
- Impetigo is a common and highly contagious bacterial skin infection, caused by Staphylococcus aureus, Streptococcus pyogenes, or both1,2
- There are two principal types of impetigo2:
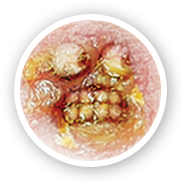
- Nonbullous (~70% of cases), causing lesions with a honey-colored crust
- Bullous (~30% of cases), causing large fluid-filled blisters
- Impetigo primarily occurs in children between the ages of 2 and 5 years, but adults and children of any age can be affected3
THERE ARE MORE THAN 3 MILLION CASES OF IMPETIGO EACH YEAR IN THE US.4
SYMPTOMATOLOGY
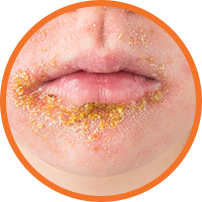
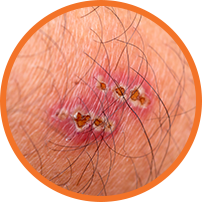
- Patients with impetigo may present with red, itchy lesions. These lesions can appear anywhere on the body but are most commonly found on exposed areas of skin (e.g., hands, arms, legs, and face around the nose and mouth)1,3
- Impetiginous lesions break open and leak a clear fluid for a few days, forming a yellowish crust1,3
PATIENT COUNSELING TIPS
To prevent impetigo from spreading, be sure your patients understand that:
Impetigo and impetiginous bacteria can easily spread through close contact with someone who is infected. It can be passed by skin-to-skin contact with unwashed hands, shared towels, sheets, clothing, toys, or other personal items1,3
It is advised that patients stay home from work or school until the infected sores have healed or following at least 24 hours of antibiotic treatment1,3


INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION AND USAGE
Xepi® (ozenoxacin) Cream, 1% is indicated for the topical treatment of impetigo due to Staphylococcus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older.
Apply a thin layer of Xepi® topically to the affected area twice daily for 5 days. Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age.
IMPORTANT SAFETY INFORMATION
Xepi®, containing 1% ozenoxacin, is for topical use only. Not for ophthalmic, oral, intranasal or intravaginal use.
Apply a thin layer of Xepi® topically to the affected area twice daily for 5 days. Affected area may be up to 100 cm2 in adult and pediatric patients 12 years of age and older or 2% of the total body surface area and not exceeding 100 cm2 in pediatric patients less than 12 years of age.
Potential for Microbial Overgrowth: Prolonged use of Xepi® may result in overgrowth of nonsusceptible bacteria and fungi. If such infections occur, discontinue use and institute alternative therapy.
Adverse reactions (rosacea and seborrheic dermatitis) were reported in 1 adult patient treated with Xepi®.
There are no available data on the use of Xepi® in pregnant women to inform a drug associated risk. No data are available regarding the presence of ozenoxacin in human milk, and the effects of ozenoxacin on the breastfed infant or on milk production.
The safety and effectiveness of Xepi® in the treatment of impetigo have been established in pediatric patients 2 months to 17 years of age. The safety profile of Xepi® in pediatric patients 2 months and older was similar to that of adults.
The safety and effectiveness of Xepi® in pediatric patients younger than 2 months of age have not been established.
Please read the US Full Prescribing Information for Xepi® available at https://www.xepicream.com/PI.
You are encouraged to report side effects of Xepi®. Please contact Biofrontera Inc. at 1-844-829-7434 or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
References: 1. Impetigo, Mayo Clinic. Accessed June 10, 2021. https://www.mayoclinic.org/diseases-conditions/impetigo/symptoms-causes/syc-20352352. 2. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90(4):229-235. 3. Impetigo: all you need to know, Centers for Disease Control and Prevention. Accessed June 10, 2021. https://www.cdc.gov/groupastrep/diseases-public/impetigo.html. 4.
How to treat impetigo and control this common skin infection. Food and Drug Administration. Accessed August 6, 2021. www.fda.gov/consumers/consumer-updates/how-treat-impetigo-and-control-common-skin-infection.